Parkinson’s Disease Models in Rats and Mice

Parkinson’s disease (PD) is a progressive nervous system disorder that affects movement [1]. Symptoms start gradually, sometimes starting with a barely noticeable tremor in just one hand. PD signs and symptoms can be different for all that are affected. Tremors are common, but the disorder also commonly causes stiffness or slowing of movement. In PD, dopaminergic neurons in the substantia nigra of the midbrain, as well as their signaling to the striatum, gradually degrade. This degradation induces an imbalance of brain activity that leads to impaired control of voluntary muscle movements. Later stages are also associated with cognitive dysfunction. The cause of PD is unknown, but several factors appear to play a role, including genes, certain toxins (e.g. MPTP) or environmental factors. In the US, about one million people are living with PD, and ~60,000 Americans are diagnosed with PD each year. Medications (e.g. L-DOPA, galantamine, etc.) and deep brain stimulation can help control the symptoms, but PD cannot yet be cured [2, 3]. Therefore, the development of more effective therapies is necessary.
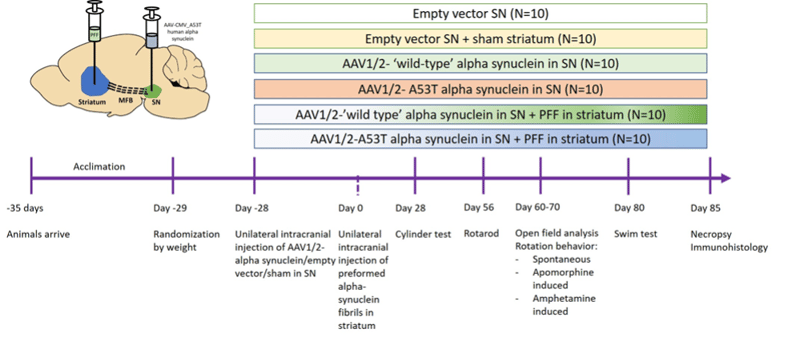
Induction: Rats or mice will be ordered at the age of ≥10 weeks old. Unilateral, intracranial injections of either 6-hydroxy dopamine (6-OHDA; 4-20 mg), adenoviral a-synuclein (AAV; 1-4 ml) or preformed a-synuclein fibrils (PFF; 5-12 mg) will be administered through a burr hole (1-2 mm in diameter) at a rate of 0.5-1 ml per minute with stereotaxic methods. The injections lead to lesions in brain areas involved in PD (e.g. striatum, medial forebrain bundle or substantia nigra).
After injection, the needle will stay in place for 5 minutes to let the substance diffuse. The needle will be withdrawn, and the animal will be removed from the stereotact. The incision will be sutured closed, and the animal will be administered 0.05 mg/kg buprenorphine XR (SC; extended release formulation) to mitigate any surgery-related pain. The animal will then be removed from anesthesia and placed on a heating pad until it regains its righting reflex. The entire procedure lasts 10-15 minutes, and the animals will be maintained and monitored for 2 weeks after their surgeries.
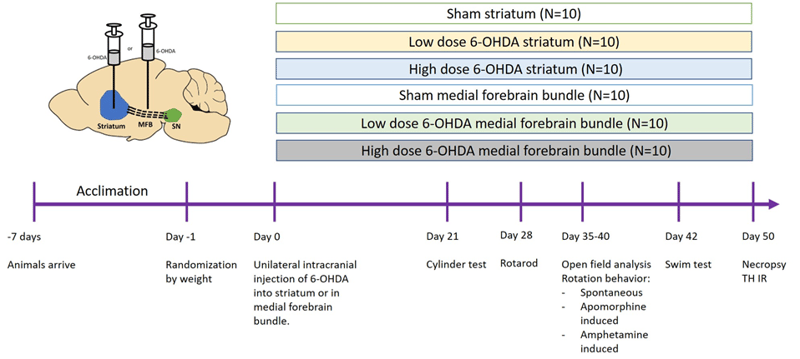
Figure 1: Example study design using 6-OHDA lesions in the striatum or medial forebrain bundle (MFB) in either mice or rats. SN = substantia nigra.
Disease Parameters & Clinical Assessment: Any combination of the below:
- Cylinder test: Animals are placed in a transparent plexiglass cylinder (16.5 cm tall and 25 cm diameter), and video recorded under red light conditions. A mirror was positioned behind the cylinder to allow a view of all forelimb movements along the wall of the cylinder. Animals remained in the cylinder until at least 30 forelimb movements were made (approximately 2 min). A forelimb wall movement was counted when the rat made a weightbearing forelimb placement on the wall of the cylinder while it was in an upright position on its hindlimbs. An investigator blind to experimental condition viewed the videos at half-speed and scored the first 20 left, right, or both forelimb wall placements. Results for each animal will be recorded as percent of contralateral forelimb use and graphed by treatment group. Percentage of contralateral forelimb use for each rat will be calculated using the formula: [(contra +1/2 both) divided by (ipsi+ contra + both)] multiplied by 100%.
- Rotarod- SOP AR-60.01 – a test for motor coordination deficits that are typically seen 1-2 months after PD-induction in rodents. Animals are placed on a rotating drum that can be set to a constant or accelerating rotation speed, and the time to fall off the drum is recorded. Acclimation and training typically occurs 1-3 days prior to assessment, depending on customer preference.
- Light/Dark Box- Animals will be acclimated to the light/dark box testing room for 1 hour. The light dark boxes have 2 compartments; 2/3 of the box (310mm x 310mm x 240mm) is brightly lit (650 lux) and 1/3 of the box (200mm x 310mm x 240mm) is dark (5 lux). Animals will be placed into the light portion the boxes and allowed to freely explore the box for 20 minutes (10min for baseline randomization). Automated tracking software will record the time spent in each zone (light vs. dark) as well as the number of transitions between each zone (door 100mm x 100mm).
- Open Field- SOP AR-63.01 – locomotor activity and anxiety. Rodents display a natural aversion to brightly lit open spaces, but they also have a drive to explore potentially threatening stimuli. Therefore, decreased levels of anxiety are correlated with increased exploratory behavior, especially the center of the arena.
- Gait Testing- SOP AR-17.03 – depending on the location of the lesion, differences in weight bearing on the left and right sides of the body may occur. These differences can be detected by inking the animals’ feet and analyzing the placement of their paws as they walk through a corridor lined with paper. Alternatively, automated determination of weight distribution can be performed in a static environment with a Bioseb dynamic weight bearing machine.
- Apomorphine- and amphetamine-induced rotation behavior: Mice are placed in cylinders with a diameter of 11.5 cm and height of 14 cm, while rats are placed in a circular maze of 44 cm width and 40 cm heigh, and allowed to habituate undisturbed for 45 min before injection with either d-amphetamine (5 mg/kg and 10 mg/kg IP or apomorphine hydrochloride (0.1 mg/kg-4 mg/kg SC). Animals are then monitored for either 90 min (d-amphetamine-induced rotations) or 45 min (apomorphine-induced rotations), due to the difference in the metabolism of the two drugs. Each subject is scored for full body rotations in 1-min intervals, every 6 min, and the net ipsilateral rotation (total right − total left 360◦turns) per minute is used as the primary dependent variable. Animals are given at least 7 days to recover following each amphetamine dose and at least 4 days following each apomorphine dose to ensure that the drugs had been eliminated from the system prior to any other behavioral testing.
- Elevated Plus Maze- SOP AR-65.01 – The elevated plus maze is best known as a measure of anxiety or a mix of anxiety and fear of heights (acrophobia). Animals that spend more time on the unprotected “open” arm during a 5-minute session are deemed less anxious.
- (Forced) Swim Test [12]- Swim test was carried out in plastic containers 40 cm length×25 cm width×16 cm height. The depth of water was 12cm and the temperature was maintained 22–25°C. Each mouse was scored in 1-min for 3 times with an interval of 10 mins. The score was a modification of that used by Marshall and Berrios: continuous swimming, 3; occasional floating, 2.5; floating time >50% of testing, 2; occasional swimming, 1.5; occasional swimming using hind limbs while floating on one side, 1; hind part sinks with head floating, 0. The observer was blind to the tested mice.
- Barnes Maze- SOP AR-64.01 – time to find the escape hole over 4 (rats) or 8 (mice) training days, memory probe of time spent in the vicinity of target escape hole 1 week (rats) or 1 day (mice) after training, reversal learning
- Morris Water Maze- SOP AR-66.01 – time to find the hidden platform over 4 (rats) or 8 (mice) training days, memory probe of time spent in the correct quadrant 1 week (rats) or 1 day (mice) after training, reversal learning
- Contextual Fear Conditioning (mice only)- SOP AR-93.01- time spent in a “frozen” posture in a learned fear response to a cue or environment
Histopathological Assessment: H&E for lesion size(s)
References
- Kouli, A., K.M. Torsney, and W.L. Kuan, Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis, inParkinson’s Disease: Pathogenesis and Clinical Aspects, T.B. Stoker and J.C. Greenland, Editors. 2018: Brisbane (AU).
- Zahoor, I., A. Shafi, and E. Haq, Pharmacological Treatment of Parkinson’s Disease, in Parkinson’s Disease: Pathogenesis and Clinical Aspects, T.B. Stoker and J.C. Greenland, Editors. 2018: Brisbane (AU).
- Dallapiazza, R.F., et al., Considerations for Patient and Target Selection in Deep Brain Stimulation Surgery for Parkinson’s Disease, in Parkinson’s Disease: Pathogenesis and Clinical Aspects, T.B. Stoker and J.C. Greenland, Editors. 2018: Brisbane (AU).
- Thakur, P., et al., Modeling Parkinson’s disease pathology by combination of fibril seeds and alpha-synuclein overexpression in the rat brain. Proc Natl Acad Sci U S A, 2017. 114(39): p. E8284-E8293.
- Bagga, V., S.B. Dunnett, and R.A. Fricker, The 6-OHDA mouse model of Parkinson’s disease – Terminal striatal lesions provide a superior measure of neuronal loss and replacement than median forebrain bundle lesions.Behav Brain Res, 2015. 288: p. 107-17.
- Batassini, C., et al., Striatal Injury with 6-OHDA Transiently Increases Cerebrospinal GFAP and S100B. Neural Plast, 2015. 2015: p. 387028.
- Boix, J., T. Padel, and G. Paul, A partial lesion model of Parkinson’s disease in mice–characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behav Brain Res, 2015. 284: p. 196-206.
- Patterson, J.R., et al., Time course and magnitude of alpha-synuclein inclusion formation and nigrostriatal degeneration in the rat model of synucleinopathy triggered by intrastriatal alpha-synuclein preformed fibrils.Neurobiol Dis, 2019. 130: p. 104525.
- Zhang, B., et al., Stereotaxic Targeting of Alpha-Synuclein Pathology in Mouse Brain Using Preformed Fibrils.Methods Mol Biol, 2019. 1948: p. 45-57.
- Song, L.K., et al., Targeted Overexpression of alpha-Synuclein by rAAV2/1 Vectors Induces Progressive Nigrostriatal Degeneration and Increases Vulnerability to MPTP in Mouse. PLoS One, 2015. 10(6): p. e0131281.
- Deumens, R., A. Blokland, and J. Prickaerts, Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol, 2002. 175(2): p. 303-17.
- Haobam, R., et al., Swim-test as a function of motor impairment in MPTP model of Parkinson’s disease: a comparative study in two mouse strains. Behav Brain Res, 2005. 163(2): p. 159-67.
Related Pages
- Glioblastoma Models in Mice
- Epilepsy Models in Rats and Mice
- Addiction Models in Rats and Mice
- Cognition Models in Rats and Mice
- Experimental Autoimmune Encephalomyelitis (EAE)
- Developmental Milestones Aka Functional Observation Battery
- Anxiety Models in Rats and Mice
- Depression Model in Rat
- Fragile X Syndrome (FXS) in Mice
- Parkinson’s Disease Models in Rats and Mice
- Alzheimer’s Disease Model in Mice

