Developmental Milestones aka Functional Observation Battery

Developmental milestones are key assessments of brain development during human infancy. Such milestones incorporate neural reflexes that become stronger, more competent, or more sophisticated as the central and peripheral nervous systems become more mature. Disruptions in brain development from gestational or perinatal exposure to stress, infection or toxins can often result in the absence, delayed acquisition, prolonged presence, or reappearance later in life of a given milestone.1-5 Furthermore, these shifts are commonly associated with developmental disabilities in humans. Thus, it is important to use a battery of rodent testing that can mimic human milestones to reduce the likelihood of developmental toxicity from any novel therapeutics.
Developmental test batteries in rodents have provided important scientific insights. For example, insults to the maternal immune system during early gestation can lead to developmental disorders such as autism spectrum disorder and schizophrenia. Maternal infection has also been associated with bipolar disorder, major depression, epilepsy, and cerebral palsy in the offspring. Thus, it appears that it is the maternal immune response, and not a specific pathogen, that elevates the risk for some neurodevelopmental disorders.6-9
Test batteries that incorporate the manifestation (or lack of) motor behaviors can also be used to pinpoint specific gestational or perinatal periods that a compound can impact nervous system development. Indeed, rats exposed to ethanol prenatally exhibit reduced grip strength and increased attentional demand, which are analogous to some characteristics of children with fetal alcohol syndrome and attention deficit disorders.10
Induction:
Pregnant dams will be ordered to be at embryonic day 4 upon arrival. Dams will typically habituate for at least 5 days before any treatments start, but some may start immediately upon arrival depending on customer preference. Pups will be cross-fostered at postnatal day 2 to control for potential differences in maternal care, and studies can last up to postnatal day 90.
An example study would involve 8 pregnant rats and up to 96 offspring. The endpoints (described below) coupled with a need for balanced litter sizes throughout the study call for a minimum of 60 pups for a single comparison between a control and test article (N=30 per arm).
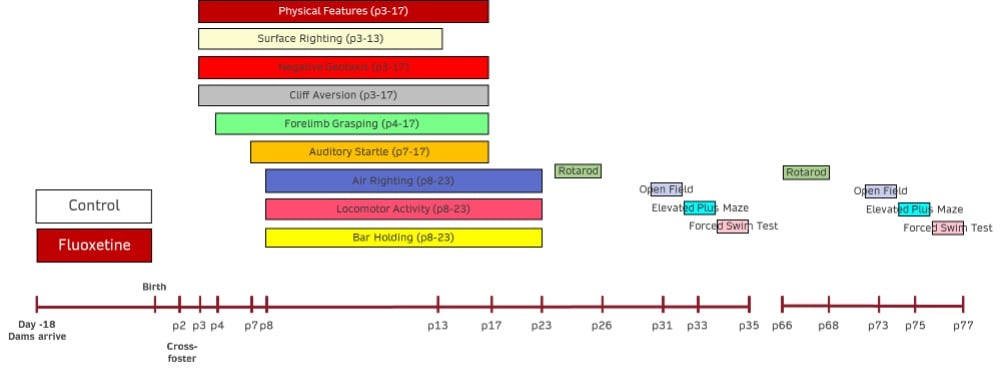
Disease Parameters & Clinical Assessment:
After induction, the animals can be tested in any combination of the following assays:
- Development of Physical Features– Each pup will be assessed from postnatal day 1 until the following physical features have been observed: sex, hair growth, ear opening, and eye opening. The postnatal day at which each characteristic is observed in each animal will be recorded.
- Surface Righting– The surface righting response of each pup will be assessed from postnatal day 1 through postnatal day 13. The time in seconds for pups placed supine to return to the prone position with all 4 paws on the ground will be recorded. The response will be limited to a maximum of 60 seconds with all non-responding animals receiving a score of 60 seconds. The postnatal day of first successful performance within the allotted time will also be recorded for each animal.
- Negative Geotaxis– The negative geotaxis response of each pup will be assessed from postnatal day 2 through postnatal day 17. The time in seconds for pups placed head down on a 30º incline to turn 90º and begin to crawl up the slope will be recorded. The response will be limited to a maximum of 30 seconds with all non-responding animals receiving a score of 30 seconds. The postnatal day of first successful performance within the allotted time will also be recorded for each animal.
- Cliff Aversion– The cliff aversion response of each pup will be assessed from postnatal day 2 through postnatal day 17. The time in seconds for pups placed with forepaws and snout over the edge of a lab bench to turn and begin to crawl away from the edge will be recorded. The response will be limited to a maximum of 30 seconds with all non-responding animals receiving a score of 30 seconds. The postnatal day of first successful performance within the allotted time will also be recorded for each animal.
- Forelimb Grasping– The forelimb grasping response of each pup will be assessed from postnatal day 4 through postnatal day 17. The time in seconds for pups to remain suspended after grasping the barrel of a blunted, 14g needle (or a similarly thin rod) will be recorded. The response will be limited to a maximum of 30 seconds with all non-responding animals receiving a score of 0 seconds. The postnatal day of first successful performance, with the criterion of remaining suspended for a minimum of 1 second, will also be recorded for each animal.
- Auditory Startle– The auditory startle response of each pup will be assessed from postnatal day 7 through postnatal day 17. The response will be determined by the presence (score = 1) or absence (score = 0) of a rapid (<1s), involuntary jump after a 25-cent coin is dropped from a height of 12 inches to a point that is 10 inches from the pup. The postnatal day of the first positive response will also be recorded for each animal.
- Air Righting– The air righting response of each pup will be assessed from postnatal day 8 through postnatal day 23. The pups will be released in a supine position from a height of approximately 60 centimeters. Their ability (score = 1) or inability (score = 0) to turn right-side up and land on all 4 paws on a bed of shavings will be determined. The postnatal day of the first positive response will also be recorded for each animal.
- Locomotor Activity– The locomotor activity of each animal will be assessed from postnatal day 8 through postnatal day 23. The time in seconds for pups placed in the center of a circle (13-cm diameter) to move outside the perimeter of that circle (all 4 paws outside) will be recorded. The response will be limited to a maximum of 30 seconds with all non-responding animals receiving a score of 30 seconds. The postnatal day of first successful performance within the allotted time will also be recorded for each animal.
- Bar Holding– The bar holding response of each animal will be assessed from postnatal day 8 through postnatal day 23. The pups will be held by the nape of the neck, and their forepaws will be placed on a wooden rod (1-cm diameter) suspended 50 centimeters above a bed of cage litter. The time in seconds for the pups to grasp and then remain on the rod will be recorded. The response will be limited to a maximum of 120 seconds with all non-responding animals receiving a score of 0 seconds. The postnatal day of first successful performance, with the criterion of remaining suspended for a minimum of 1 second, will also be recorded for each animal.
- Rotarod– an assay of motor function on a rotating drum that is determined on postnatal days 23-25 and 35.
- Morris Water Maze– an assay of learning, memory and cognitive function that is determined on postnatal days 38-49.
Biochemical Assessment:
Corticosterone, BDNF, serotonin, norepinephrine, melatonin, cytokines
References
- Nguyen, A.T., Armstrong, E.A., and Yager, J.Y. (2017) Neurodevelopmental reflex testing in neonatal rat pups. J Vis Exp 122: 55261. doi: 10.3791/55261.
- Altman, J. and Sudarshan, K. (1975) Postnatal development of locomotion in the laboratory rat. Anim Behav 23: 896-920.
- Wu, J.Y. Henins, K.A., Gressens, P., Gozes, I., Fridkin, M.. Brenneman, D.E., and Hill, J.M. (1997) Neurobehavioral development of neonatal mice following blockade of VIP during the early embryonic period. Peptides 18(8): 1131-7. doi: 10.1016/s0196-9781(97)00146-0.
- Sima, A., and Sourander, P. (1978) The effect of pre- and postnatal undernutrition on axonal growth and myelination of central motor fibers. Acta Neuropath 42:15-18.
- Salas, M. (1972) Effects of early malnutrition on the development of the swimming ability in the rat. Physiol Behav8: 119-122.
- Bergdolt, L. and Dunaevsky, A. (2019) Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog Neurobiol 175: 1–19. doi: 10.1016/j.pneurobio.2018.12.002.
- Suresh, A., and Dunaevsky, A. (2017) Relationship between synaptic AMPAR and spine dynamics: impairments in the FXS mouse. Cereb cortex 27(8): 4244–4256. doi: 10.1093/cercor/bhx128.
- Pendyala, G., Chou, S., Jung, Y., Coiro, P., Spartz, E., Padmashri, R., Li, M., and Dunaevsky, A. (2017) Maternal immune activation causes behavioral impairments and altered cerebellar cytokine and synaptic protein expression. Neuropsychopharmacology 42(7): 1435–1446. doi:org/10.1038/npp.2017.7.
- Coiro, P., Padmashri, R., Suresh, A., Spartz, E., Pendyala, G., Chou, S., Jung, Y., Meays, B., Roy, S., Gautam, N., Alnouti, Y., Li, M., and Dunaevsky, A. (2015) Impaired synaptic development in a maternal immune activation mouse model of neurodevelopmental disorders. Brain Behav Immun 50: 249–258. doi:10.1016/j.bbi.2015.07.022.
- Brys, I., Pupe, S., and Bizarro, L. (2014) Attention, locomotor activity and developmental milestones in rats prenatally exposed to ethanol. Int J Dev Neurosci 38: 161–168. doi:10.1016/j.ijdevneu.2014.08.007.
Related Pages
- Glioblastoma Models in Mice
- Epilepsy Models in Rats and Mice
- Addiction Models in Rats and Mice
- Cognition Models in Rats and Mice
- Experimental Autoimmune Encephalomyelitis (EAE)
- Developmental Milestones Aka Functional Observation Battery
- Anxiety Models in Rats and Mice
- Depression Model in Rat
- Fragile X Syndrome (FXS) in Mice
- Parkinson’s Disease Models in Rats and Mice
- Alzheimer’s Disease Model in Mice

