Your Preclinical to Clinical LC-MS Bioanalysis Division

Our pharmaceutical and bioanalytical team consists of Principal Investigators and Scientists with an average of 15+ years of experience in non-regulated and regulated bioanalysis with a focus on method development to accelerate your compound’s validation.
You will also have full access to our Sample Management team to support the analysis of your studies in compliance with your in-life study, a Metrology team who can support in-house mass spectrometry maintenance, and a full Quality Assurance team with an impeccable 40+ year regulatory track record.
Bioanalytical Services Nonregulated discovery bioanalysis
 As a proud winner of the Frost & Sullivan 2022 North American Bioanalytical Testing Services Customer Value Leadership Award, we are committed to delivering more attention, more insight, and a superlative client experience to help you advance to your next milestone.
As a proud winner of the Frost & Sullivan 2022 North American Bioanalytical Testing Services Customer Value Leadership Award, we are committed to delivering more attention, more insight, and a superlative client experience to help you advance to your next milestone.
To talk to an expert about our Regulated Bioanalytical Services, please click here.
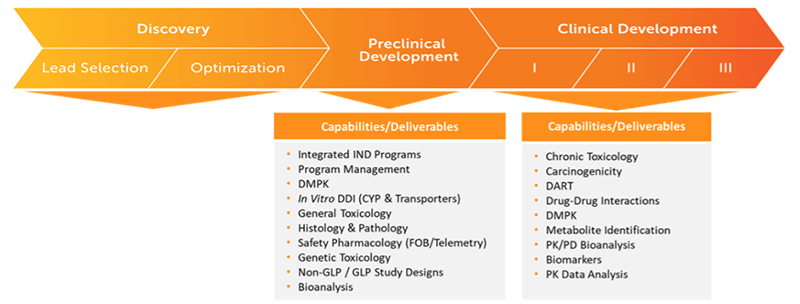
Pharmaceutical Capabilities
- Sample Management and Sample Storage
- Metrology and Instrument Qualification
- Dose Formulation Analysis (DFA)
- Stability Testing
- In Vitro Bioequivalence Analysis
- Bioavailability Analysis
Sample Analysis Types
- Bioanalytical Support for Domestic and International Studies
- Preclinical and Clinical Studies for Safety and Efficacy
- Immunogenicity
- Bioequivalence
- Pharmacokinetics
- Phoenix® WinNo
- Noncompartmental analysis
Speciality Compounds
- Small Molecules by LC-MS/MS
- Proprietary and Nonproprietary Drugs
- Prodrugs
- Insecticides
- Synthetic analogues
- Inhibitors
- Metabolites
- Chemotherapeutics
- Anesthetics
- Antiandrogens
- Hormones
- Immunosuppressives
- Vaccines
- Large Molecules or Biologics by LC-MS/MS
- Proteins
- Peptide(s)
- Oligonucleotides
- Nucleic acids
Sampling Techniques or Systems
- Microdialysate
- In Vitro:
- Hepatoc
- Media
Bioanalytical Methods List
Inotiv’s bioanalytical solution supports your program from preclinical research through clinical development. We are here to help you by delivering actionable insights with on-time results to move you to the next milestone. Our GLP nonproprietary methods have been validated in accordance with U.S. FDA Crystal City and current regulatory guidelines. You’ll benefit from our expert scientific team and state-of-the-art technologies to support your investigation into drug-drug interactions, concomitant drugs, and generics studies.
To talk to an expert about our nonproprietary methods, please click here.
Contemporary Platforms
- Liquid Chromatography, Tandem Mass Spectrometry
- AB SCIEX™ Platform (4000, 5500, 6500, 7500)
- Bio-Automation
- TomTec Quadra 96 Liquid Handling System
- Hamilton Microlab STAR Liquid Handling System
- TubeWriter™ 360
- SPEX SamplePrep 2010 Geno/Grinder Cell Lyser and Homogenizer
- HPLC and UPLC systems:
- Shimadzu Nexera®
- Agilent®
- Waters™
- CTC and Shimadzu Multi-Plate Autosamplers
- UV-Vis, fluorescence, electrochemical, charged aerosol detectors
- SpectraMax® M5 Microplate Readers (Vis, UV, Fl)
Information Systems
- Watson LIMS™ 7.5 SP1
- Projects Database System – Project Life Cycle Management
- Chromeleon™ Data Acquisition for HPLC
- Sciex Analyst®
- SoftMax® Pro GxP
- PerkinElmer® ELN
- MasterControl™ Document/Training Management System
- 21 CFR Part 11 Compliant
