
Now Available!
2024 Product Guide
Receive a copy of our current research models and services product guide.
Quantify any protein, in any sample, with any modification, without antibodies
Target Sufficiency® enables direct, quantitative analysis of drug target systems in cells, biofluids, tissues, and tissue models, including formalin-fixed, paraffin-embedded (FFPE) tissues.
Supporting your critical research with our inflation reduction model program
Reduce costs with our complimentary sample for evaluation in your lab.
In vivo solutions for Developmental and Reproductive Toxicology and Juvenile Animal Studies
Count on our expertise in IBD animal models for your next in vivo study
Sample our new Biosecure Plus™ SOPF models
Discover our array of highly characterized PDX models
Expect More From Your Discovery, Development and Research Models Partner
Explore Our Full Offering of Services and Products

Discovery and Development
Inotiv delivers a broad array of nonclinical and analytical services from discovery through clinical development.
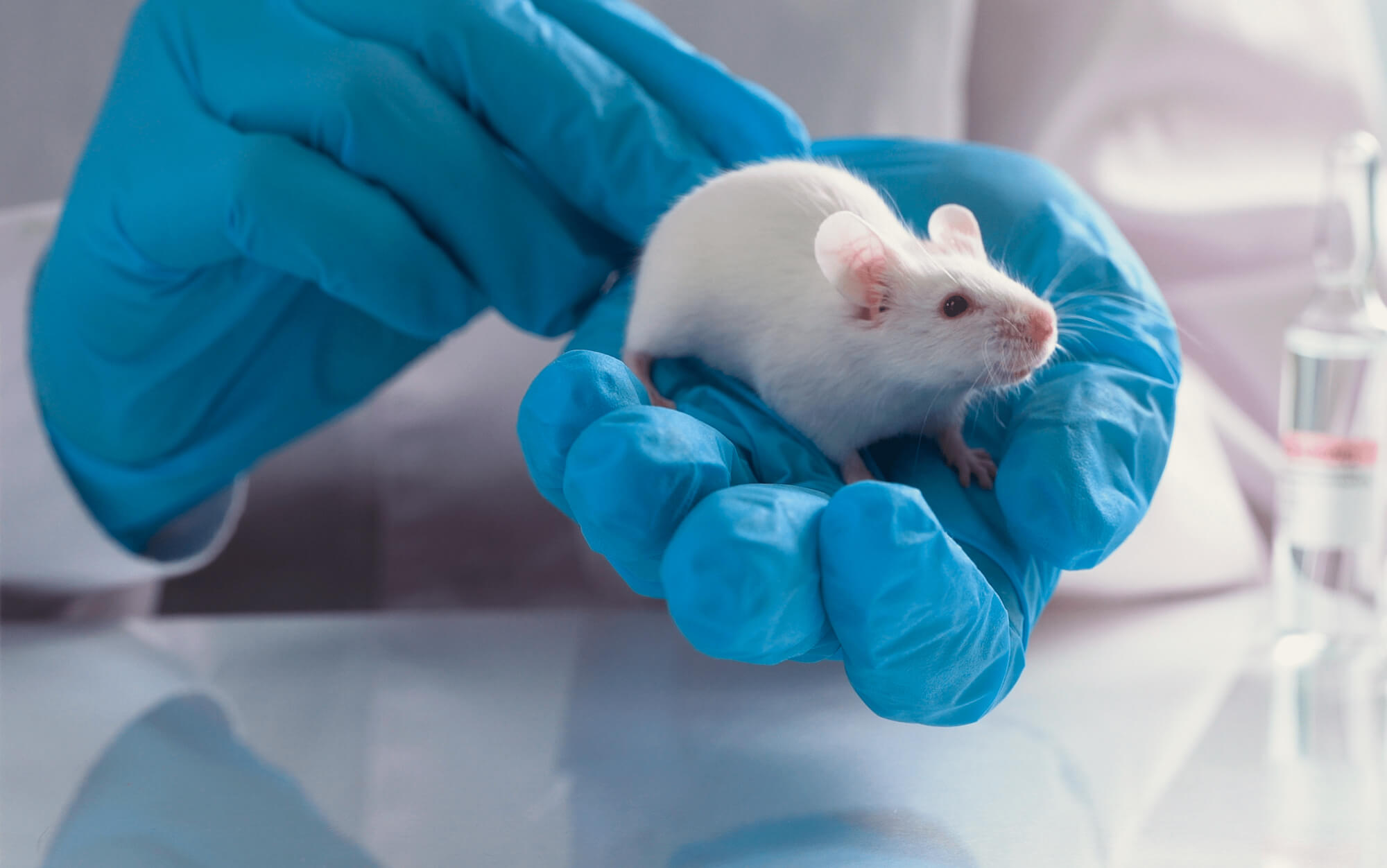
Research Models and Services
Inotiv provides the broadest range of research models and related services to pharmaceutical and biotech companies, government, academia, and other life science organizations.
The Latest From Inotiv
Webinar: Longitudinal Clinical Observations and Motor Coordination Assessments of the SOD1G93A Rat Model of Amyotrophic Lateral Sclerosis (ALS)
July 22, 2024 | 11:00am EDT Presented by: Dr. Jessica Ramadhin – Field Applications Scientist, Taconic Biosciences,
Webinar: Bridging the Gap: Uncovering Predictive Biomarkers from IBD Patients in Preclinical Animal Models
July 24, 2024 | 2:00pm EDT Presented by: Dr. Melissa M. Walker, Associate Director of Pharmacology – Gastrointestinal Inflammation,
EUROTOX
September 8-11, 2024 Copenhagen, Denmark Booth #15 EUROTOX is the Federation of European Toxicologists and European Societies of Toxicology, which tog...
Inotiv, Inc. to Report Fiscal 2024 Third Quarter Financial Results and Host Conference Call on Thursday, August 8, 2024
July 25, 2024 WEST LAFAYETTE, Ind., July 25, 2024 (GLOBE NEWSWIRE) -- Inotiv, Inc. (NASDAQ: NOTV) (the “Company”, or “Inotiv”),&...
Inotiv, Inc. Provides Business Updates
June 10, 2024 WEST LAFAYETTE, Ind., June 10, 2024 (GLOBE NEWSWIRE) -- Inotiv, Inc. (Nasdaq: NOTV) (the “Company” or “Inotiv”),
Inotiv Reaches Agreement to Resolve Previously-Announced Investigation into Shuttered Cumberland Facility
Company reaffirms commitment to maintaining appropriate standards of animal welfare June 3, 2024 WEST LAFAYETTE, Ind., June 03, 2024 (GLOBE NEWSWIRE) -- Inotiv Inc. – ...
On-demand Webinar
Not All IBD Models Are Created Equally
How Differences Between Experimental IBD Models Can Impact Your Drug Development Program
Scientific Poster
Comparative Evaluation of Different Plating Methodologies and Sources of Induced S9 Metabolic Activation on the Mutagenic Potential of N-Nitrosodipropylamine Using the Ames Test
Due to the current FDA concern and guidance on reducing the risk of Nitrosamine impurities in human drugs, there has been an increased industry need for testing of these impurities using the Bacterial Reverse Mutation (Ames) test. As Nitrosamines are not readily detectable by standard Ames methodologies, specifically cited in the Ames testing guideline (OECD 471), the need for a robust protocol better suited for this class of compounds is warranted.

