Indomethacin Induced Crohn’s in Rats

Induction of Indomethacin Induced Crohn’s in Rats:
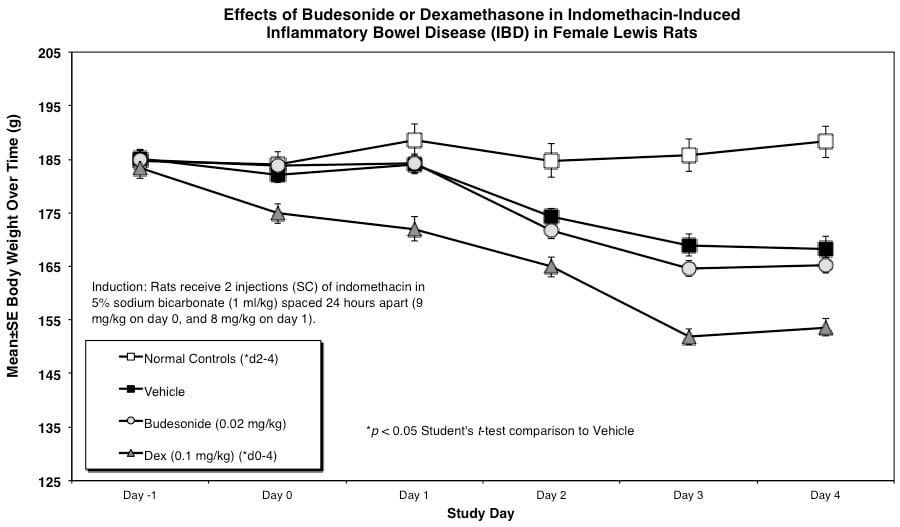
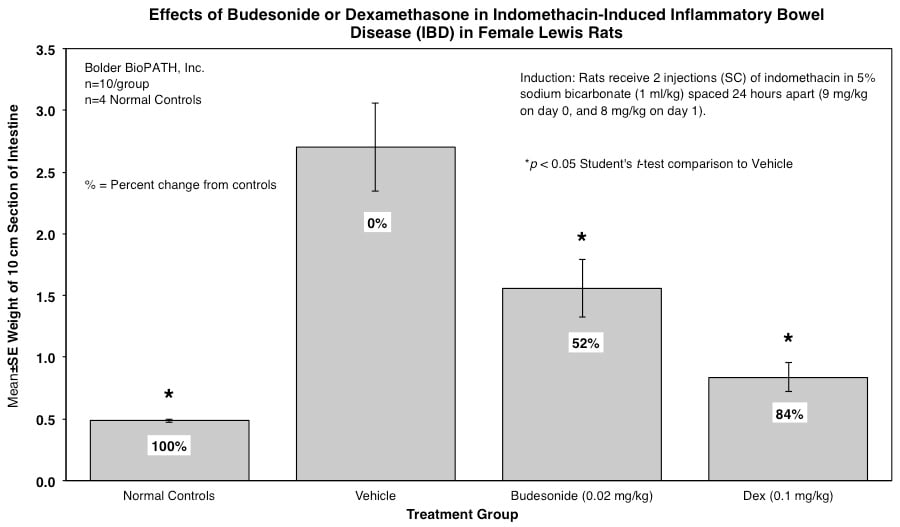
Rats receive two injections (SC) of indomethacin in 5% sodium bicarbonate (1 ml/kg) spaced 24 hours apart (9 mg/kg on day 0, and 8 mg/kg on day 1).
Disease Parameters/Progression:
Onset of disease occurs rapidly once indomethacin is administered. Lesions appear as longitudinal ulcers mostly on the mesenteric side of the distal small intestine with varying degrees of inflammation, and necrosis that is apparent in all layers of the intestinal wall. A single SC injection (7.5 mg/kg) has been shown to produce acute injury and inflammation that resolves within one week, whereas two daily injections result in both acute and chronic injury with more extensive epithelia injury and persistent elevated mucosal permeability.1
Dosing Paradigms:
Dosing is initiated prior to or on the day of indomethacin administration (Day 0), and is continued until study termination.
- Route of administration: SC, PO, IP, IV, IC
Clinical Assessment:
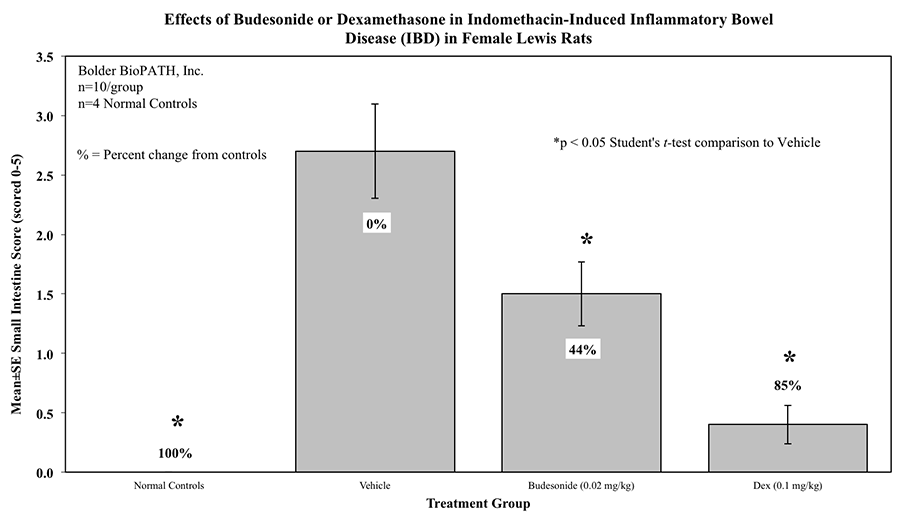
Rats are weighed daily (study days 0 through 4). At necropsy, animals are euthanized and a 10 cm area of small intestine (area at risk for indomethacin injury) is removed and weighed.
Intestines of animals that survive until the final study day are scored for gross lesions as follows:
Gross Scoring Criteria
0 = Normal.
0.5 = Very Minimal thickening, multifocal in area at risk.
1 = Minimal thickening, fairly diffuse in area at risk.
2 = Mild to moderate small intestinal/mesenteric thickening throughout area at risk.
3 = Moderate thickening with one or more definite adhesions that would be easy to separate.
4 = Marked thickening with numerous hard to separate adhesions.
5 = Severe intestinal lesion resulting in death.
Histopathological Assessment:
Tissues are examined microscopically by a board certified veterinary pathologist (Dr. Alison Bendele) and scored according to these methods.
Sample Data (Click on image to enlarge):
VIEW MORE INFORMATION IN PDF FORM HERE
Subcutaneous (SC) injections of indomethacin induce a severe enteropathy in rats that resembles human Crohn’s disease.2 Administration of indomethacin increases mucosal permeability and produces injury and inflammation in the small intestine; however, the mechanisms by which this occurs are not fully understood. Enterohepatic circulation of indomethacin is thought to be important in promoting the acute phase of the disease, while endogenous microflora are thought to play a role in perpetuating and intensifying the chronic phase.1 Compounds, such as anti-TNFa, that are effective in the treatment of human IBD, have activity in this model and it is being used to investigate potential new therapies.3
Optional Endpoint
- PK/PD blood collections
- Cytokine/chemokine analysis via Luminex(R)
- Other sandwich ELISAs
- CBC/clinical chemistry analysis
- Soft tissue collection
- Histopathologic analysis
- Immunohistochemistry analysis
- Endoscopy
References
- Yamada T, Deitch E, Specian RD, et al. Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation. 1993;17(6):641–662.
- Derelanko, MJ, Long JF. Effects of corticosteroids on indomethacin-induced intestinal ulceration in the rat. Dig Dis Sci. 1980;25(11):823–829.
- Williams, P, Bendele A, Deldar A, et al. General pharmacology of pergolide in animals. 2nd Communication: Gastrointestinal, renal and miscellaneous studies. Arzneim Forsch/Drug Res. 1992;42:891–895.
For more information about Indomethacin Induced Crohn’s in Rats, contact us here.