AAV Induced Lupus Nephritis In Mice

Induction:
Systemic Lupus Erythematosus (SLE) is an autoimmune disease in which the immune system produces antibodies to cellular components of the body, leading to widespread inflammation and tissue damage. There are numerous murine models that have long been employed in an effort to understand the cellular and genetic requirements for SLE induction. The classic models of spontaneous lupus include the F1 hybrid between the New Zealand Black (NZB) and New Zealand White (NZW) mouse strains (NZB/W F1) and its derivatives, the MRL/lpr (fas signaling deficient), and BXSB/Yaa (with a duplicated gene called toll-like receptor 7 (TLR7) that is responsible for the autoimmunity phenotype) strains. All of these models portray their own iterations of lupus-like diseases with a subset of symptoms akin to those observed in human SLE, namely, autoantibody production (antibodies to double stranded DNA (dsDNA), lymphoid activation and hyperplasia, elevated proteinuria (excess protein in the urine which is a sign of kidney damage), and lupus nephritis. All these strains reliably develop spontaneous lupus nephritis. AAV induced model is a refinement of the spontaneous lupus NZB/W F1 model. Injecting NZB mice with mouse interferon alpha (mIFNa) adeno-associated virus (AAV) vectors synchronizes the spontaneous disease state associated with NZB/W mice resulting in an acceleration of the disease (from months to weeks) and a synchronization of disease state across animals (Moser, 2009; Kono, 2006; Theofilopoulos, 1985; Rudofsky, 1993; Morel, 1994; Liu, 2013)
Disease Parameters/Progression:
NZB female mice arrive at 11 weeks of age. One week later, day 0, all mice are weighed, randomized by weight and injected with either LacZ-AAV of IFNa-AAV intravenously. The IFNa-AAV vehicle control group develops significant disease compared to LacZ-AAV vehicle group by histopathology. Cyclophosphamide and tofacitinib (100 mg/kg) treatments significantly suppress disease.
On day 0, all groups will be injected IV with one of the two AAVs. One group of non-disease control animals will be dosed with 100 uL of LacZ-AAV. The remaining groups of animals will be diseased, induced with mIFNa-AAV. These groups will be composed of at least one vehicle control group, one positive treatment control group (tofacitinib or cyclophosphamide) and a number of drug treatment groups. Treatment will start on day -1, day 0, or day +1, depending on whether prophylactic, developing or therapeutic dosing is desired.
Dosing Paradigms:
- Developing (Prophylactic) – Begin dosing on study day -1 (or earlier) and continue until necropsy on day 432.
- Semi-Established (Prophylactic) – Begin dosing on study day 0 and continue until necropsy on day 43.
- Established (Therapeutic) – Begin dosing on study day +1 (or more) and continue until necropsy on day 43.
- Route of administration: SC, PO, IP, IV
Clinical Assessment:
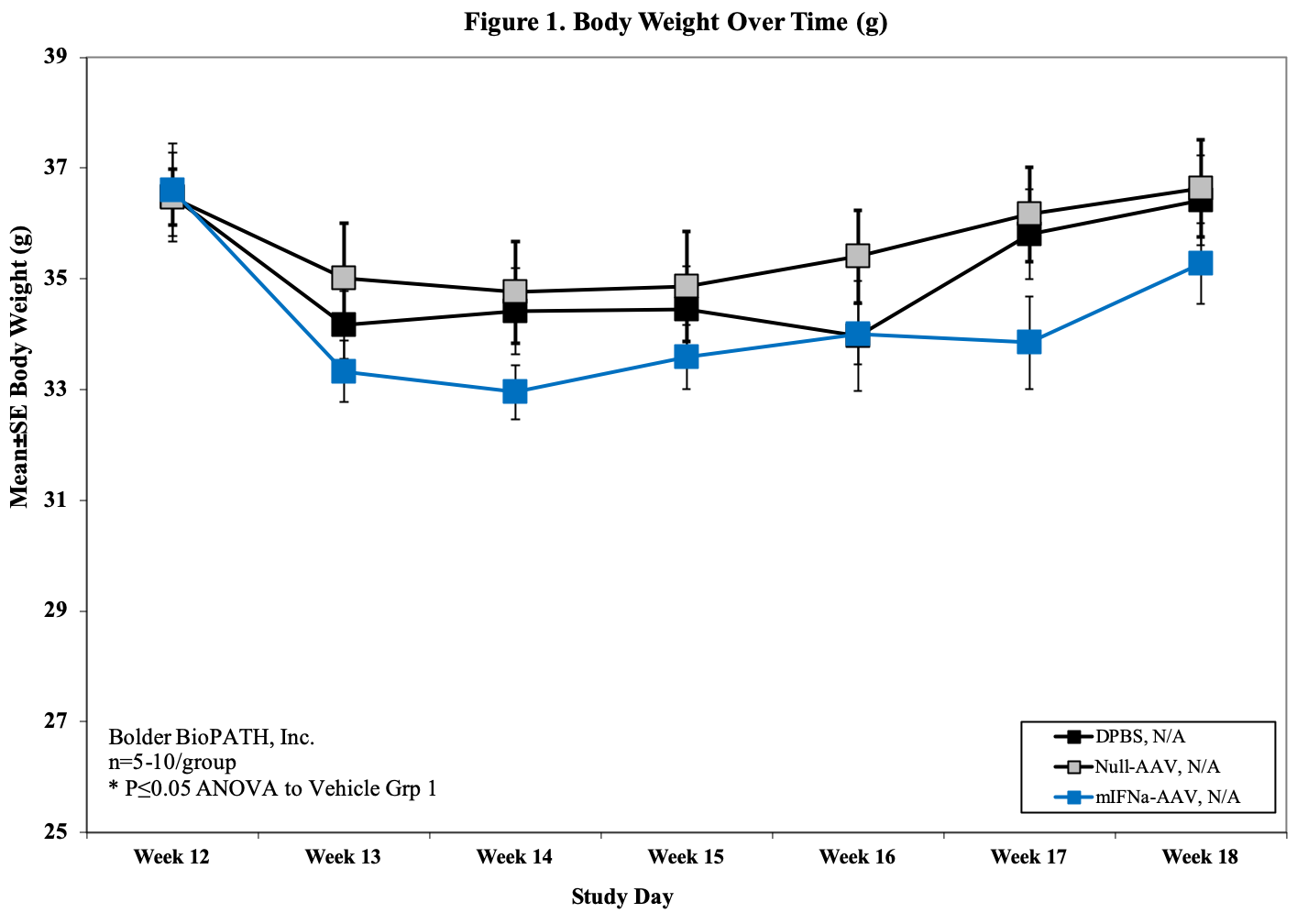
- Body weight (baseline, d7, d14, d21, 28, d35, d42)
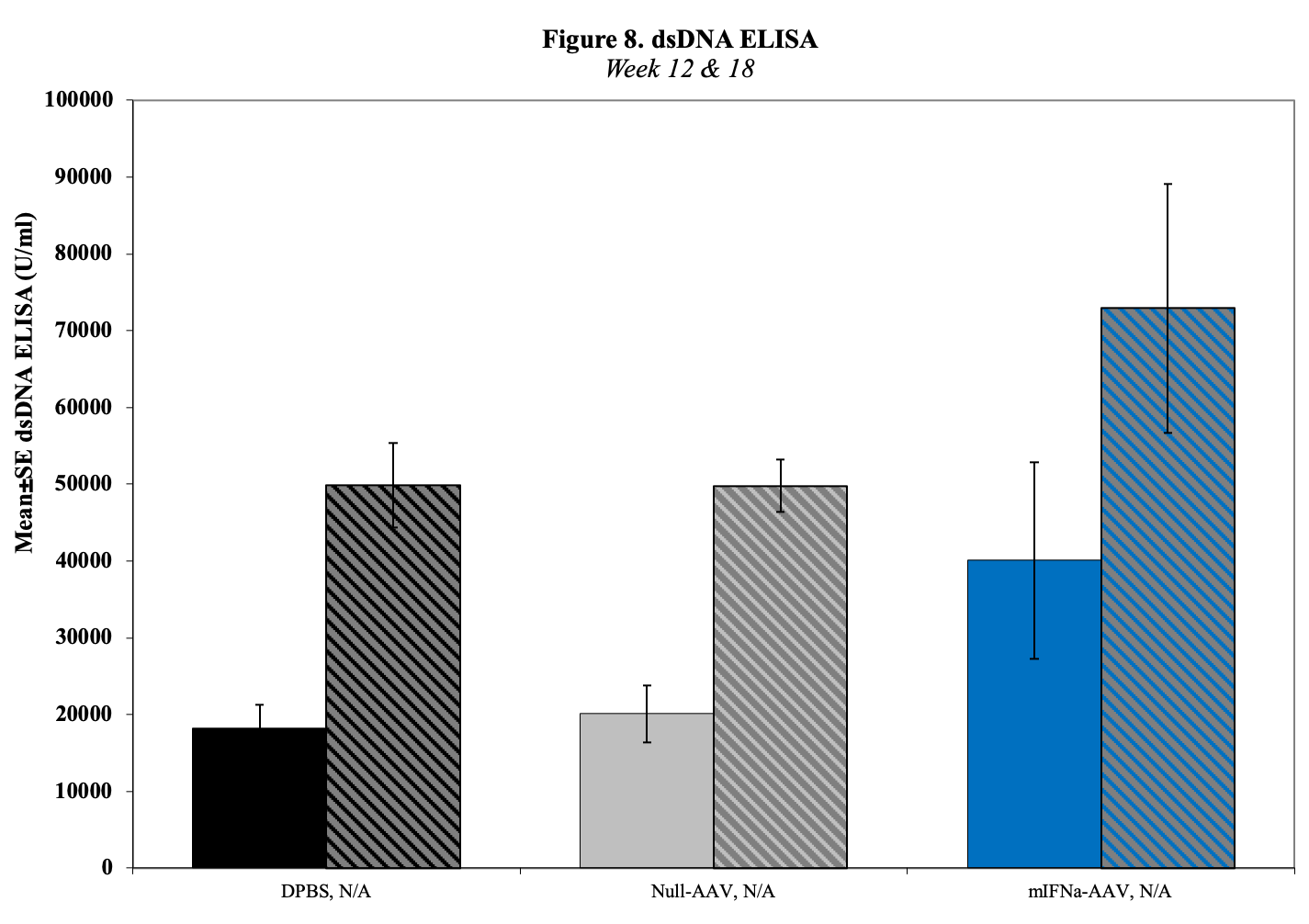
- Serum for dsDNA Titers (baseline, d14, 28, d42)
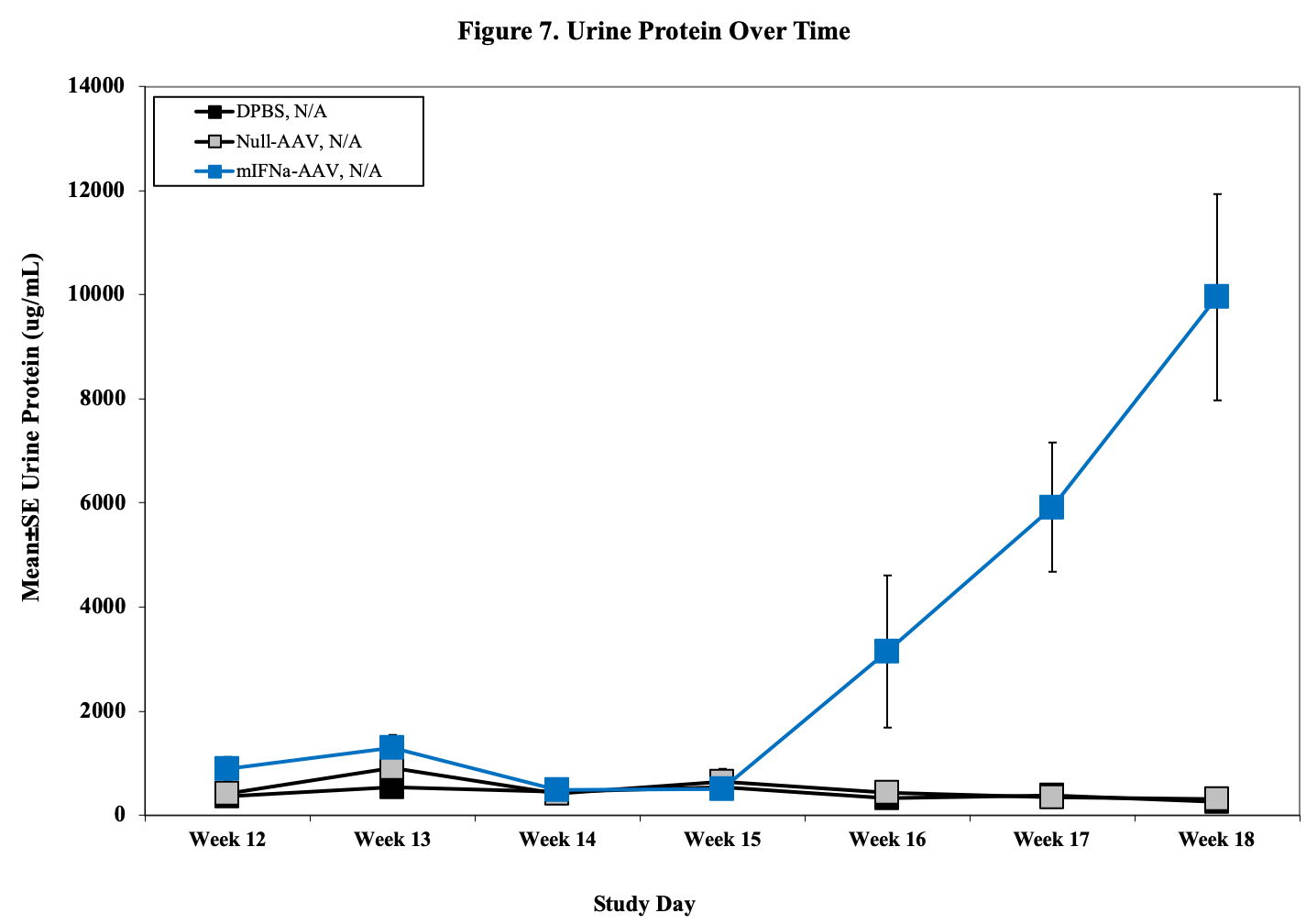
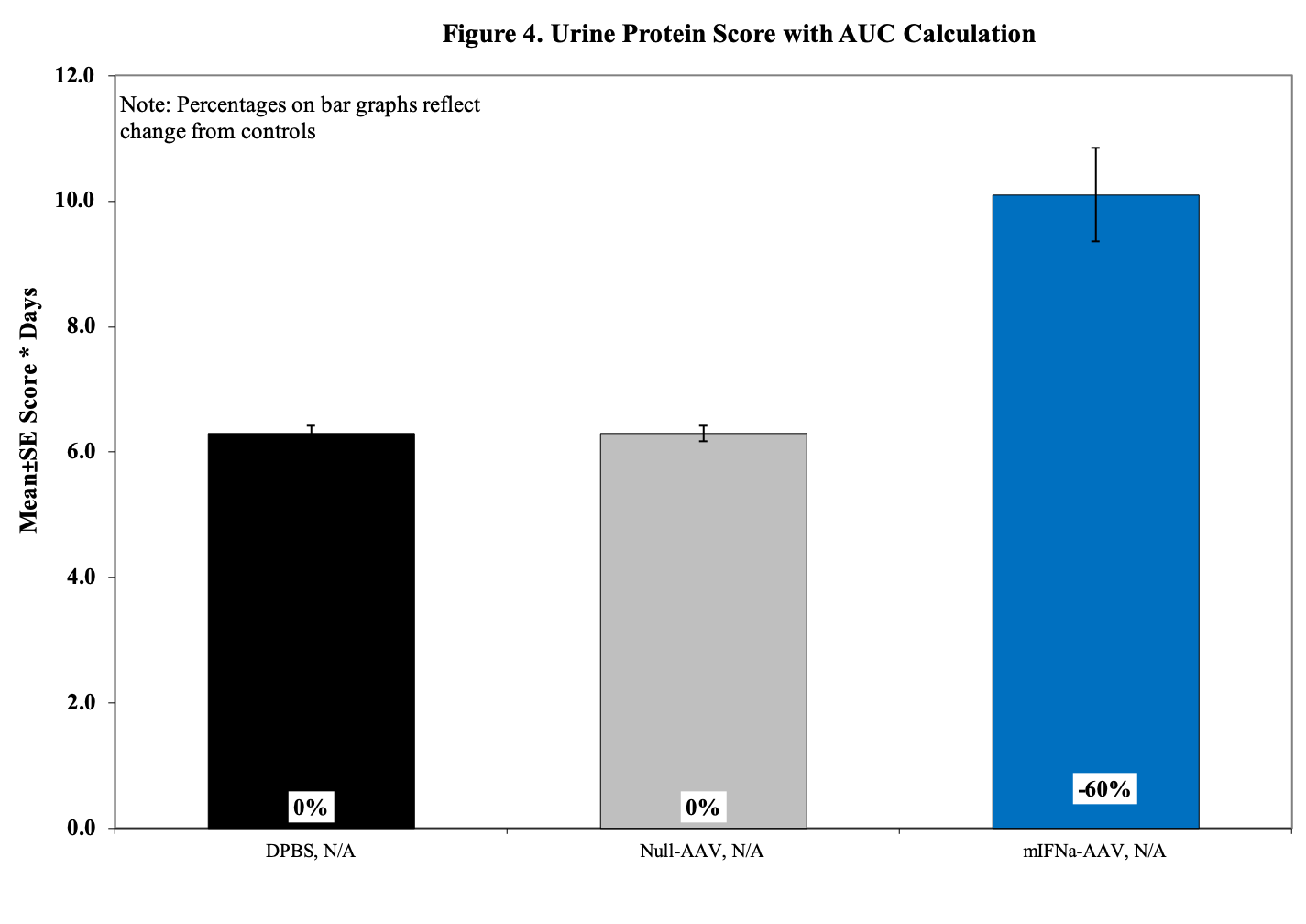
- Urine for ELISA (baseline, , d14, 28, d42)
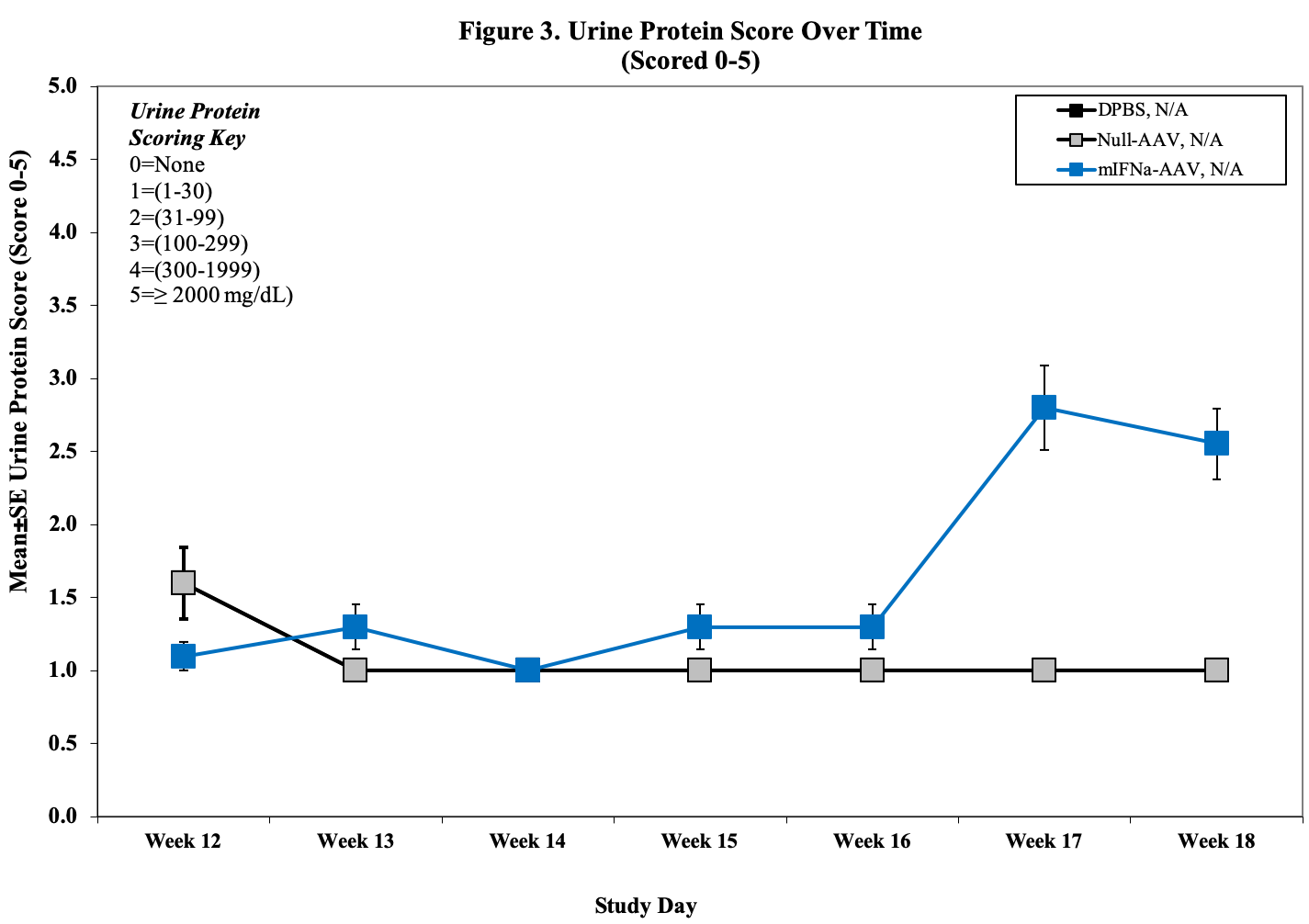
- Urine Protein Scoring Key:
0 = None.
1 = 1–30 mg/dL
2 = 31–99 mg/dL
3 = 100–299 mg/dL
4 = 300–1999 mg/dL
5 = ≥2000 mg/dL
Histopathological Assessment:
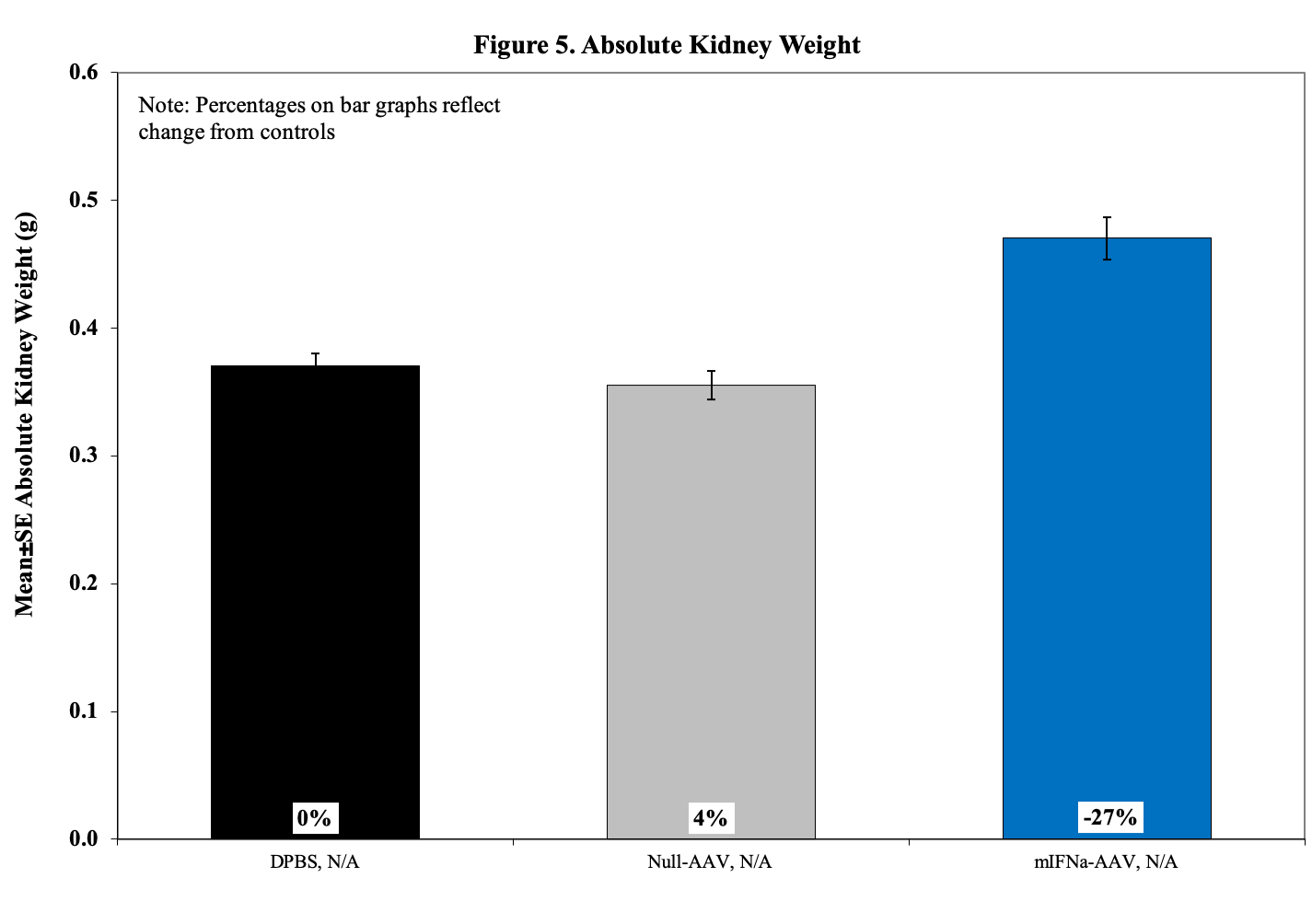
Kidneys are evaluated using H&E stained slides for characteristic histopathologic changes of lupus-induced nephritis including glomerular diameter, crescent percentage, cortical tubular protein cast severity, interstitial inflammation, and vasculitis.
Sample Data (Click on image to enlarge):
References
- Pisetsky DS, Buyon JP, Manzi S. Chapter 17. Systemic lupus erythematosus. In: Klippel JH, Crofford LJ, Stone JH, Weyand CM. Primer on the Rheumatic Diseases. Edition 12. Arthritis Foundation, Atlanta, GA., 2001.
- Rus V, Hajeer A, Hochberg MC. Chapter 7. Systemic lupus erythematosus. In: Silman AJ, Hochberg MC (eds.) Epidemiology of the Rheumatic Disease. 2nd edition. Oxford University Press, New York, 2001.
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Maradit Kremers H, and Wolfe F for the National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum 2008;58(1):26–35
- L. Moser, J. A. Kelly, C. J. Lessard, and J. B. Harley, “Recent insights into the genetic basis of systemic lupus erythematosus,” Genes and Immunity, vol. 10, no. 5, pp. 373–379, 2009.
- H. Kono and A. N. Theofilopoulos, “Genetics of SLE in mice,” Springer Seminars in Immunopathology, vol. 28, no. 2, pp. 83–96, 2006.
- Theofilopoulos and F. J. Dixon, “Murine models of systemic lupus erythematosus,” Advances in Immunology, vol. 37, pp. 269–390, 1985
- H. Rudofsky, B. D. Evans, S. L. Balaban, V. D. Mottironi, and A. E. Gabrielsen, “Differences in expression of lupus nephritis in New Zealand Mixed H-2(z) homozygous inbred strains of mice derived from New Zealand Black and New Zealand White mice: origins and initial characterization,” Laboratory Investigation, vol. 68, no. 4, pp. 419–426, 1993.
- Morel, U. H. Rudofsky, J. A. Longmate, J. Schiffenbauer, and E. K. Wakeland, “Polygenic control of susceptibility to murine systemic lupus erythematosus,” Immunity, vol. 1, no. 3, pp. 219–229, 1994.
- Canadian Council on Animal Care (1998), Guidelines on: Choosing an appropriate endpoint inexperiments using animals for research, teaching and testing. Ottawa, Canada. 2.
- Reilly CM, Oates JC, Cook JA, Morrow JD, Halushka PV, Gilkeson GS. Inhibition of mesangial cell nitric oxide in MRL/lpr mice by prostaglandin J2 and proliferator activation receptor-gamma agonists. J Immunol 2000;164:1498–1504. [PubMed: 10640767]
- Reilly CM, Farrelly LW, Viti D, Redmond ST, Hutchison F, Ruiz P, Manning P, Connor J, Gilkeson GS. Modulation of renal disease in MRL/lpr mice by pharmacologic inhibition of inducible nitric oxide synthase. Kidney Int 2002;61:839–846. [PubMed: 11849435]
- Reilly CM, Gilkeson GS. Use of genetic knockouts to modulate disease expression in a murine model of lupus, MRL/lpr mice. Immunol Res 2002;25:143–153. [PubMed: 11999168]
- Reilly CM, Olgun S, Goodwin D, Gogal RM Jr. Santo A, Romesburg JW, Ansar Ahmed S, Gilkeson GS. Interferon regulatory factor-1 gene deletion decreases glomerulonephritis in MRL/lpr mice. Eur J Immunol. 2006
- Christoph T, Kogel B, Schiene K, Meen M, De Vry J, Friderichs E. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol 2005;507:87–98. [PubMed: 15659298]
Related Pages
- Anti-GBM Serum Induced Nephritis In SVJ Mice
- AAV Induced Lupus Nephritis In Mice
- Systemic Lupus Erythematosus (SLE) In MRL/MpJ-Fas/J Mice
- Systemic Lupus Erythematosus (SLE) In NZBWF1/J Mice