Developmental and Reproductive Toxicology

 Inotiv supports DART and Juvenile animal studies (JAS) which are essential in identifying the potential risks of undesirable effects of your drug candidate, agrichemical product, or other chemical. Take advantage of our DART and JAS expertise in providing solutions that deliver the insights you need in program planning and individual study design in accordance with internationally recognized ICH and OECD guidelines. We provide a customized approach to meet your unique requirements as a complete in vivo integrated program or as a stand-alone service.
Inotiv supports DART and Juvenile animal studies (JAS) which are essential in identifying the potential risks of undesirable effects of your drug candidate, agrichemical product, or other chemical. Take advantage of our DART and JAS expertise in providing solutions that deliver the insights you need in program planning and individual study design in accordance with internationally recognized ICH and OECD guidelines. We provide a customized approach to meet your unique requirements as a complete in vivo integrated program or as a stand-alone service.
Gain the insight and expertise you need to progress to your next milestone with Inotiv’s DART and JAS service offerings
DART Services
CUSTOMIZED STUDY DESIGNS
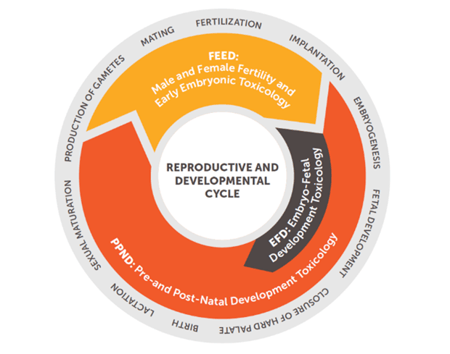
- Fertility and Early Embryonic Development studies for male and/or female (FEED, SEG I)
- Embryo-Fetal Development/prenatal developmental toxicity studies (EFD, SEG II)
- Pre- and Postnatal Developmental studies (PPND, SEG III)
- Combined 28-day/reproduction screening (OECD 421/422)
- Extended One-Generation Reproductive Toxicity studies (EOGRTS, OECD 443)
- Male and Female Rat Pubertal Assays (USEPA Endocrine Disruptor Screening Program, OPPTS 890.1450/1500)
- Rat Hershberger Assay (USEPA Endocrine Disruptor Screening Program, OPPTS 890.1400)
- Rat Uterotrophic Assay (USEPA Endocrine Disruptor Screening Program, OPPTS 890.1600)
ROUTE OF ADMINISTRATION
- Oral: Gavage, Dietary, Drinking Water
- Subcutaneous
- Intravenous
- Intramuscular
JAS Services
CUSTOMIZED STUDY DESIGNS
- Species selection and age for intended patient populations
- Consideration of timing of dosing, exposure, monitoring, pharmacological/toxicological differences
ROUTE OF ADMINISTRATION
- Oral Gavage
- Subcutaneous
- Intramuscular
SPECIES
- Mouse
- Rat
Behavioral Assessment Offering
LOCOMOTOR ACTIVITY
- Models ability to navigate environment and habituation
STARTLE RESPONSE
- Measures reflex due to a sudden stimulus for learning and habituation
MORRIS WATER MAZE
- Studies spatial learning and memory
FUNCTIONAL OBSERVATIONAL BATTERY (FOB)
- Detailed observation to detect changes in central nervous system, autonomic nervous system, and neuromuscular functions
Our experts deliver the insights you deserve to get the answers you need, whether in DMPK data analysis, biomarker analysis, or bioequivalence studies.


