Epilepsy Models in Rats and Mice

Epilepsy is not fully controlled although many therapies with diverse mechanisms have been developed.1,2 Epilepsy affects a minimum of 3 million adults and almost 500,000 children in the US alone.3 The clinical value of valium-based modulators of epilepsy is seen in that some of them have been marketed for over 50 years.4 However, these drugs, despite their efficacy, have issues with abuse/dependency liabilities and impairment of either brain function and/or muscle coordination.5 Retaining the efficacy of these drugs, but reducing the risk of using them has been the goal of epilepsy research for over 4 decades,6,7 but the goal has not been reached.
Induction:
Male rats will be ordered to be at least 90-100g and at least ~28 days old when on study, and male mice will be ordered to be at least 21-32g and at least 5-12 weeks old when on study.4 Developmental studies of infantile seizures will use all rat pups resulting from timed-pregnant animals, and the pups may be tested between postnatal days 7-70.8-12 Some clients may prefer a developmental model of infantile spasms (e.g. West syndrome) that involves treating the dams with betamethasone (0.4 mg/kg i.p) or restraint stress (5-45 minutes) delivered twice on gestational day 15.13-17 A graphical representation of a typical study design is below and would apply to any age of rat. Study designs for mice are similar except the post-induction monitoring period would only be 30 minutes.
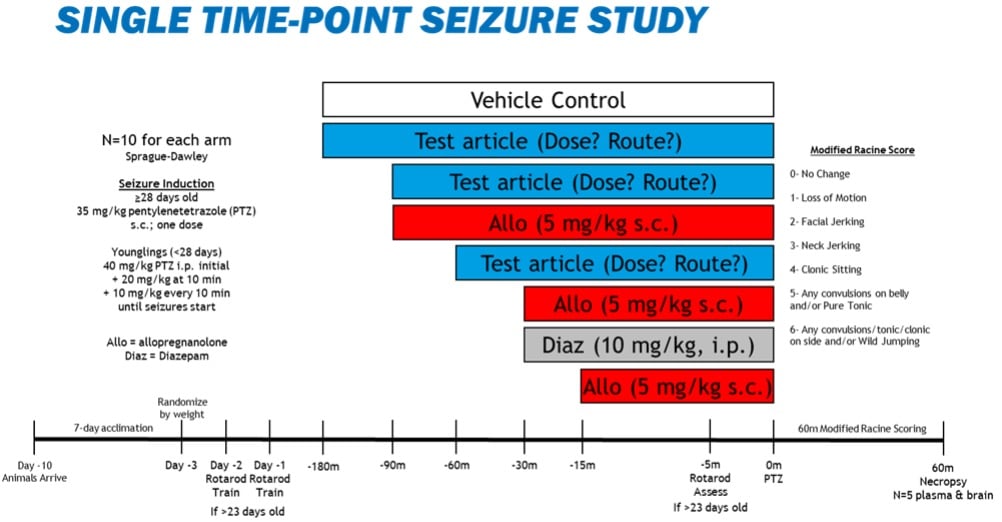
Control and Test articles will be administered prior to seizure induction. Control arms include vehicle controls that match the longest timing of the test articles, allopregnanolone (5 mg/kg SC) at 90, 30, and 15 minutes before seizure induction (separate arms for each time point), and diazepam (10 mg/kg, IP) 30 minutes prior to seizure induction.4,18 Some developmental studies may use up to 3 doses of adrenocorticotrophic hormone (ACTH; 0.1-0.4 mg/kg i.p.) as another control arm.13-17
Seizures will be induced at study time 0 by the administration of one of 4 exogenous stimuli: pentylenetetrazol SC or IP (30 mg/kg mice; 10-40 mg/kg rats),4,9-11 kainic acid IP (30 mg/kg mice; 5-30 mg/kg rats),8 N-methyl-D-aspartate (5-200 mg/kg IP depending on age),13-17 or two, 2-minute pulses of 140dB noise (fire alarm or similar placed with the animal in a sound-attenuating box; 1-minute intertrial interval).12 The animals will then be evaluated in the following tests outlined below (N=10 for each arm):
Disease Parameters & Clinical Assessment:
- Rotarod – SOP 60.01, animals, if physically mature enough (at least postnatal day 23), may be trained on study days -2 and -1 and assessed 5 minutes prior to seizure induction on study day 0.
- Modified Racine Scoring – observation/video recording of all animals for 30 minutes (mice) or 30-60 minutes (rat) after seizure induction. Scoring is as follows:
- No Change
- Loss of Motion
- Facial Jerking
- Neck Jerking
- Clonic convulsion while sitting
- Any convulsions while on belly and/or Pure Tonic convulsion
- Any convulsions/tonic/clonic that occur while animal lays on its side and/or Wild Jumping19
- Barnes Maze – SOP 64.01, animals, if physically mature enough (at least postnatal day 28), may be baselined prior to seizure induction on study day 0 and then tested again as late as postnatal day 70.
- Morris Water Maze – SOP 66.01, animals, if physically mature enough (at least postnatal day 38), may be baselined prior to seizure induction on study day 0 and then tested again as late as postnatal day 70.
Histopathological Assessment:
as requested by client; neuroprotection (H&E or neuron-specific IHC) and neuronal sprouting (Timm silver stain) in hippocampus.
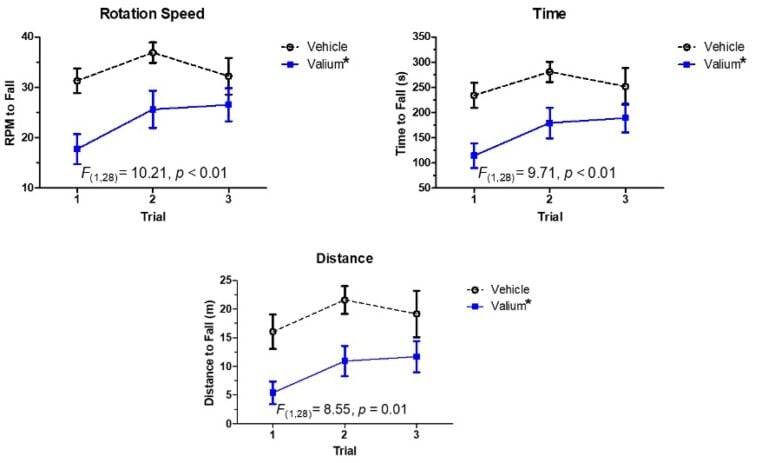
Sample Data (Click on image to enlarge):
The rotarod can detect significant side effects of anti-epileptic drugs. Diazepam is quite effective at reducing seizures and anxiety, but it also impacts motor control in rodents on the rotarod (and on generally functioning humans). Animals on valium (10 mg/kg IP, 30 minutes before assay) tend to fall off of a rotating object more quickly than saline-injected animals, and these effects on coordination should be avoided.
References
- Guerreiro, C.A. (2016) Epilepsy: is there hope? Indian J Med Res 144(5): 657-660.
- Shih, J.J., Whitlock, J.B., Chimato, N., Vargas, E., Karceski, S.C. and Frank, R.D. (2017) Epilepsy treatment in adults and adolescents: expert opinion. Epilepsy Behav 69: 186e222.
- Zack, M.M. and Kobau, R. (2017) National and state estimates of the numbers of adults and children with active epilepsy – United States, 2015. MMWR 66: 821–825.doi: 10.15585/mmwr.mm6631a1
- Witkin, J.M., Smith, J.L., Ping, X., Gleason, S.D., Poe, M.M., Li, G., Jin, X., Hobbs, J., Schkeryantz, J.M., McDermott, J.S., Alatorre, A.I., Siemian, J.N., Cramer, J.W., Airey, D.C., Methuku, K.R., Tiruveedhula, V., Jones, T.M., Crawford, J., Krambis, M.J., Fisher, J.L., Cook, J.M. and Cerne, R. (2018) Bioisosteres of ethyl 8-ethynyl-6-(pyridin-2-yl)-4H-benzo[f]imidazo [1,5-a][1,4]diazepine-3-carboxylate (HZ-166) as novel alpha 2,3 selective potentiators of GABAA receptors: Improved bioavailability enhances anticonvulsant efficacy. Neuropharmacology137: 332-343. doi:10.1016/j.neuropharm.2018.05.006
- Drummer, O.H. (2002) Benzodiazepines – effects on human performance and behavior. Forensic Sci Rev 14(1-2): 1-14.
- Klepner, C.A., Lippa, A.S., Benson, D.I., Sano, M.C. and Beer, B. (1979) Resolution of two biochemically and pharmacologically distinct benzodiazepine receptors. Pharmacol Biochem Behav 11(4): 457-462.
- Skolnick, P. (2012) Anxioselective anxiolytics: on a quest for the holy grail. Trends Pharmacol Sci 33(11): 611-620.
- Albala, B.J., Moshé, S.L. and Okada, R. (1984) Kainic-acid-induced seizures: a developmental study. Brain Res315(1): 139-148. doi:10.1016/0165-3806(84)90085-3
- Auvin, S., Dozières-Puyravel, B., Avbersek, A., Sciberras, D., Collier, J., Leclercq, K., Mares, P., Kaminski, R.M. and Muglia, P. (2020). Radiprodil, a NR2B negative allosteric modulator, from bench to bedside in infantile spasm syndrome. Ann Clin Transl Neurol 7(3): 343-352. doi:10.1002/acn3.50998
- el Hamdi, G., de Vasconcelos, A.P., Vert, P. and Nehlig, A. (1992) An experimental model of generalized seizures for the measurement of local cerebral glucose utilization in the immature rat. I. Behavioral characterization and determination of lumped constant. Dev Brain Res 69(2): 233-242. doi:10.1016/0165-3806(92)90164-r
- Hussenet, F., Boyet, S. and Nehlig, A. (1995) Long-term metabolic effects of pentylenetetrazol-induced status epilepticus in the immature rat. Neurosci 67(2): 455-461. doi:10.1016/0306-4522(95)00062-n
- Thomas, A.M., Bui, N., Perkins, J.R., Yuva-Paylor, L.A. and Paylor, R. (2012) Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacol 219(1): 47-58. doi:10.1007/s00213-011-2375-4
- Iacobas, D. A., Iacobas, S., Chachua, T., Goletiani, C., Sidyelyeva, G., Velíšková, J., and Velíšek, L. (2013) Prenatal corticosteroids modify glutamatergic and GABAergic synapse genomic fabric: insights from a novel animal model of infantile spasms. J Neuroendocrinol 25(11): 964–979. doi:10.1111/jne.12061
- Velísek, L., Chachua, T., Yum, M. S., Poon, K. L., and Velísková, J. (2010) Model of cryptogenic infantile spasms after prenatal corticosteroid priming. Epilepsia 51 Suppl 3(Suppl 3): 145–149. doi:10.1111/j.1528-1167.2010.02630.x
- Galanopoulou A. S. (2013) Basic mechanisms of catastrophic epilepsy — overview from animal models. Brain Dev35(8): 748–756. doi:10.1016/j.braindev.2012.12.005
- Wang, J., Wang, J., Zhang, Y., Yang, G., Zhou, W. J., Shang, A. J., and Zou, L. P. (2012) Proteomic analysis of adrenocorticotropic hormone treatment of an infantile spasm model induced by N-methyl-D-aspartic acid and prenatal stress. PloS one 7(9): e45347. doi:10.1371/journal.pone.0045347
- Wang, Y. J., Zhang, Y., Liang, X. H., Yang, G., and Zou, L. P. (2012) Effects of adrenal dysfunction and high-dose adrenocorticotropic hormone on NMDA-induced spasm seizures in young Wistar rats. Epilepsy res 100(1-2): 125–131. doi:10.1016/j.eplepsyres.2012.02.001
- Reddy, D.S. and Rogawski, M.A. (2001) Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia 42(3): 337-344.
- Lüttjohann, A., Fabene, P.F. and van Luijtelaar, G. (2009) A revised Racine’s scale for PTZ-induced seizures in rats. Physiol Behav 98(5): 579–586. doi:10.1016/j.physbeh.2009.09.005
- Stegmayr, C., Surges, R., Choi, C.H., Burda, N., Stoffels, G., Filß, C., Willuweit, A., Neumaier, B., Heinzel, A., Shah, N.J., Mottaghy, F.M. and Langen, K. J. (2020) Investigation of cerebral O-(2-[18F]fluoroethyl)-L-tyrosine uptake in rat epilepsy models. Mol Imaging Biol 22(5): 1255-1265. doi:10.1007/s11307-020-01503-x
Related Pages
- Glioblastoma Models in Mice
- Epilepsy Models in Rats and Mice
- Addiction Models in Rats and Mice
- Cognition Models in Rats and Mice
- Experimental Autoimmune Encephalomyelitis (EAE)
- Developmental Milestones Aka Functional Observation Battery
- Anxiety Models in Rats and Mice
- Depression Model in Rat
- Fragile X Syndrome (FXS) in Mice
- Parkinson’s Disease Models in Rats and Mice
- Alzheimer’s Disease Model in Mice