Dextran Sulfate Induced Colitis (DSS) in Mice

Induction:
Mice are exposed to Dextran Sulfate Salt (DSS) in water for 5 or 7 days to induce inflammation and gland loss with erosion in the colon. Acute DSS-induced mucosal injury is dependent on the administered DSS water concentration but not on the consumed DSS dose, and minor variations in fluid consumption do not affect the severity of DSS-induced injury in mice.1
Disease Parameters/Progression:
Mice have been shown to develop acute colitis with signs of diarrhea, gross rectal bleeding, and body weight loss within 6 to 10 days after ingesting DSS.2 Gross and histopathologic changes resulting from this treatment resemble those occurring in human ulcerative colitis, a subset of inflammatory bowel disease.2,3,4 Early histologic changes include progressive loss of the crypt resulting in erosions by day 5. The earliest changes are very focal and not associated with inflammation. Inflammation occurs secondarily after erosions appear; acute clinical symptoms (diarrhea, bloody stool) are associated with the presence of erosions and inflammation. Following DSS treatment, mice develop a colitis characterized by areas of activity (erosions and inflammation), inactivity, crypt distortion, florid epithelial proliferation and possible dysplasia3.
Dosing Paradigms:
- Dosing is initiated prior to or once exposure to DSS begins and continues until study termination.
- Route of administration: SC, PO, IP, IV, IC
Clinical Assessment:
Mice are weighed and water consumption is measured daily. At necropsy, the entire colon from each mouse is removed and the length is determined. The colon contents are scored for gross changes using the following criteria:
0 = Normal
1 = Few formed pellets to semi-solid stool
2 = Semi-solid to fluid stool with or without definite evidence of blood
3 = Bloody stool
4 = Bloody fluid
5 = No content
Only animals that survive to study termination are included in the analysis of terminal colon lengths and scores.
Histopathological Assessment:
Colon sections are examined microscopically by a board certified veterinary pathologist (Dr. Alison Bendele) and scored (as proximal colon, distal colon, and total colon) according to these methods.
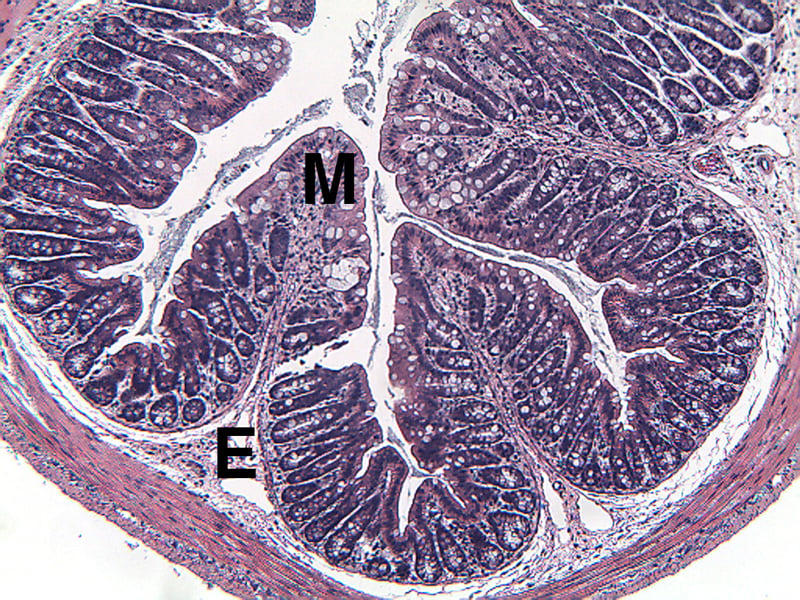
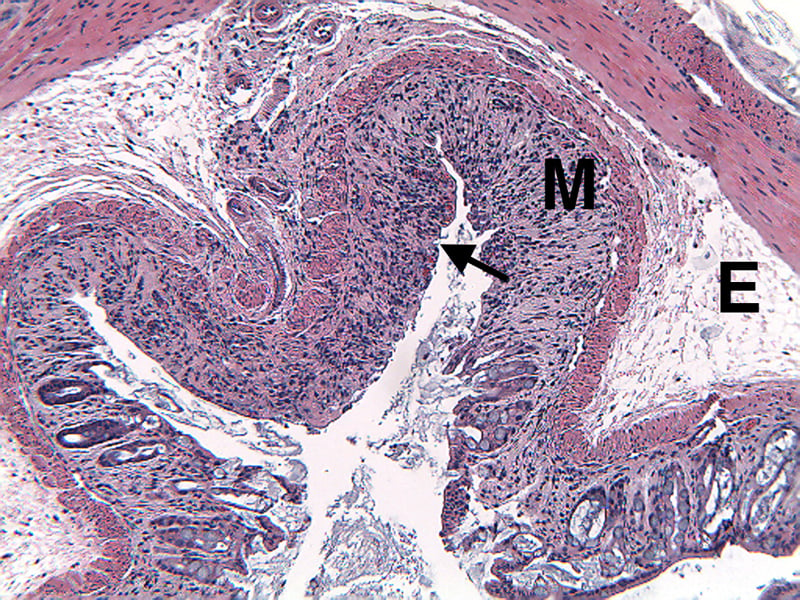
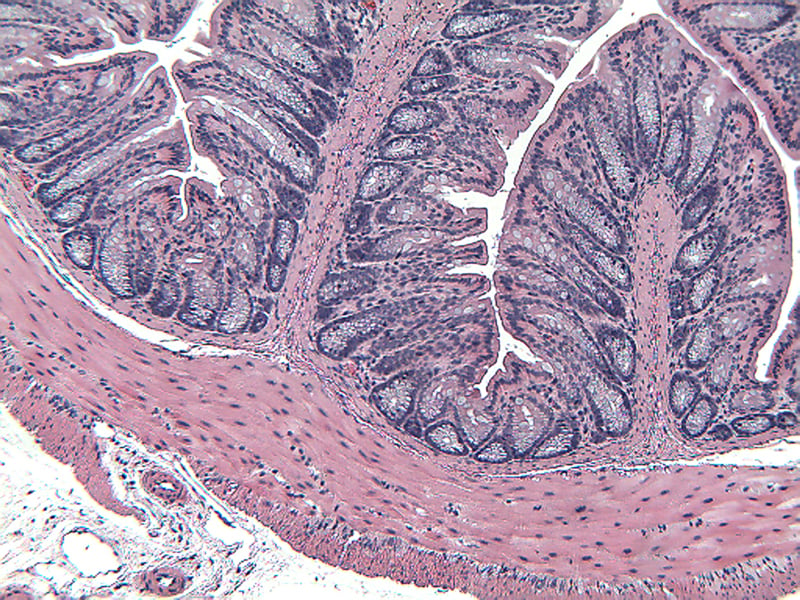
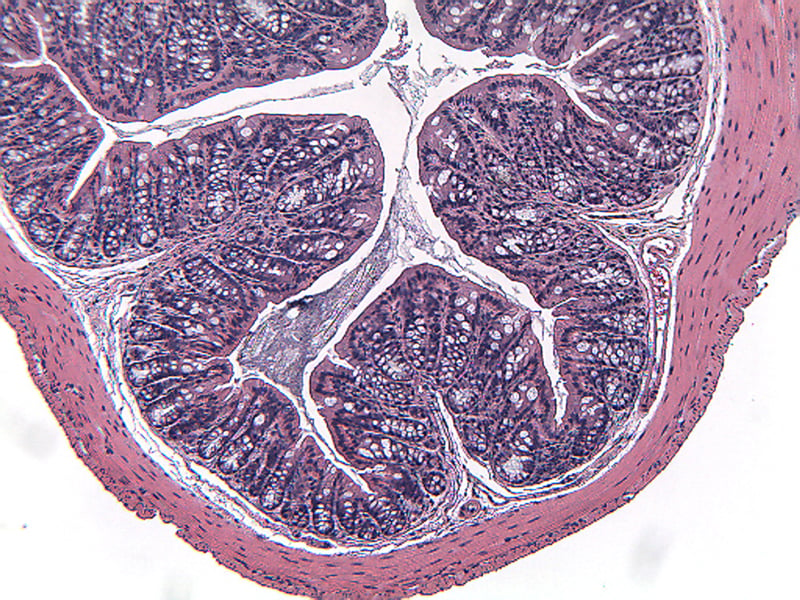
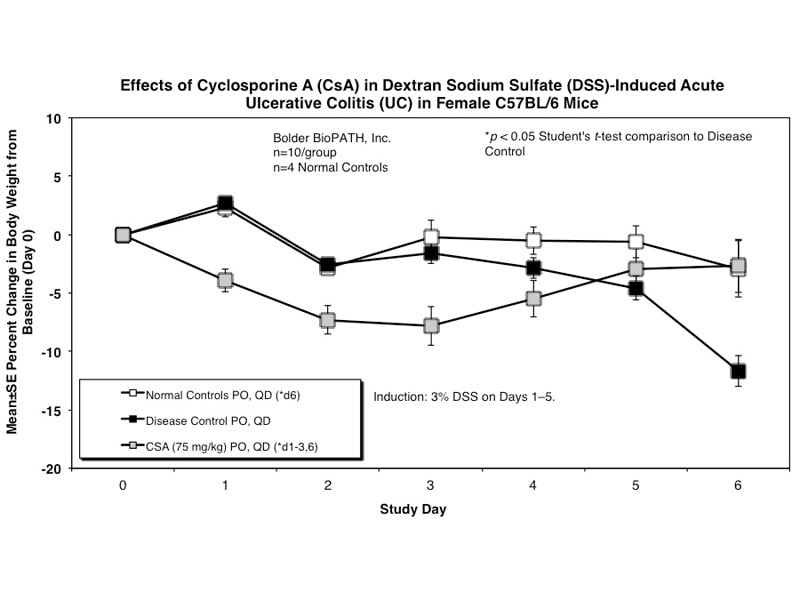
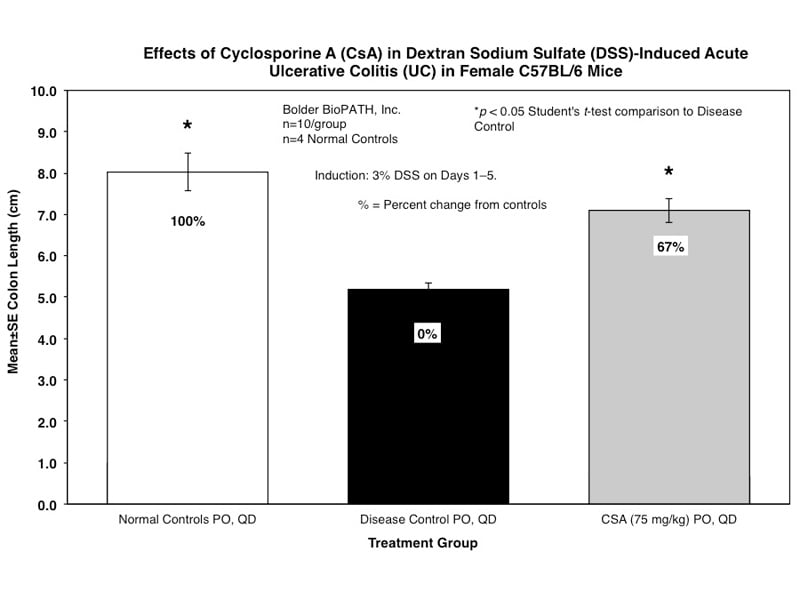
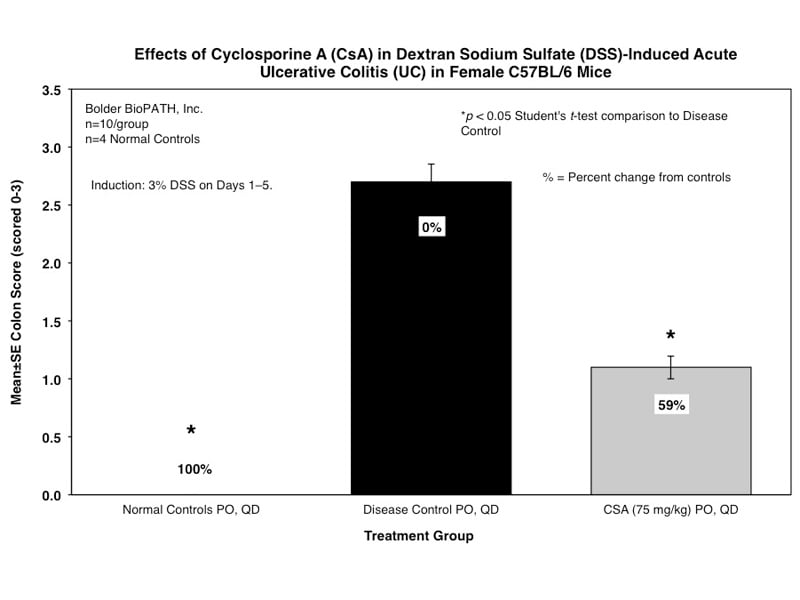
Sample Data (Click on image to enlarge):
Notes:
Compounds that are effective in the treatment of human IBD have activity in this model and it is being used to investigate potential new therapies.1,5,6
Optional Endpoint
- PK/PD blood collections
- Cytokine/chemokine analysis via Luminex(R)
- Other sandwich ELISAs
- CBC/clinical chemistry analysis
- Soft tissue collection
- Histopathologic analysis
- Immunohistochemistry analysis
- Disease Activity Index (DAI)
- Endoscopy
References
- Egger B, Bajaj-Elliott M, et al. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion, 2000;62(4):240–248.
- Okayasu I, Hatakeyama S, Yamada M, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterol 1990; 98(3):694–702.
- Cooper HS, Murthy SNS, Shah RS and Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest, 1993;69:238–249.
- Animal Models of Intestinal Inflammation in “Inflammatory Bowel Disease” ed MacDermott RP and Stenson WF. Elsevier, New York; 1992.
- Axelsson LG, Landstrom E, Bylund-Fellenius AC. Experimental colitis induced by dextran sulphate sodium in mice:beneficial effects of sulphasalazine and olsalazine. Aliment Pharmacol Ther 1998; 12:925–934.
- Miceli R, Hubert M, Santiago G, Yao D, Coleman T, Huddleston KA and Connolly K. Efficacy of keratinocyte growth factor-2 in dextran sulfate sodium-induced murine colitis. J Pharmacol Exp Ther 1999; 290: 464–471.
For more information about Dextran Sulfate Induced Colitis (Mouse) contact us.