Plato BioPharma, an Inotiv company, provides collaborative preclinical study design to support project goals through the systematic development, validation and application of preclinical disease model and assay systems within core therapeutic areas.

Plato BioPharma, Inc. was founded in 2010 by Dr. Craig Plato who built a world-class preclinical drug discovery CRO specializing in in vivo physiological and pharmacological studies with complimentary biochemical, biomarker and histology analysis in the cardiovascular, renal, pulmonary, diabetes and hepatic therapeutic areas.
Prior to founding PBI, Dr. Plato led in vivo pharmacology for Myogen, Inc.; when it was acquired by Gilead Sciences in 2005, Dr. Plato expanded the in vivo laboratory and grew core competencies in cardiovascular and renal research. In 2009, Gilead consolidated the cardiovascular operation with a second company it acquired and outsourced in vivo operations. Dr. Plato obtained financial backing, acquired a significant amount of Gilead’s laboratory equipment and leased the lab facility. As a result, Dr. Plato continued the science in the state-of-the art laboratory he designed and built for his two previous employers under the new name Plato BioPharma, Inc.
Dr. Plato assembled an esteemed Board of Directors and strategic advisory board as well as an experienced staff with expertise in small animal surgery and in vivo pharmacology. PBI has continued to grow in both capacity and expertise, providing integrated pre-clinical in vivo CRO services to an expanding number of clients in pharmaceutical and biomedical research. Inotiv is pleased to incorporate the talents and experience of Plato BioPharma into its portfolio of world-class drug discovery and development services.
To learn more about the acquisition, read our news release.
In Vivo Pharmacology
Therapeutic Areas
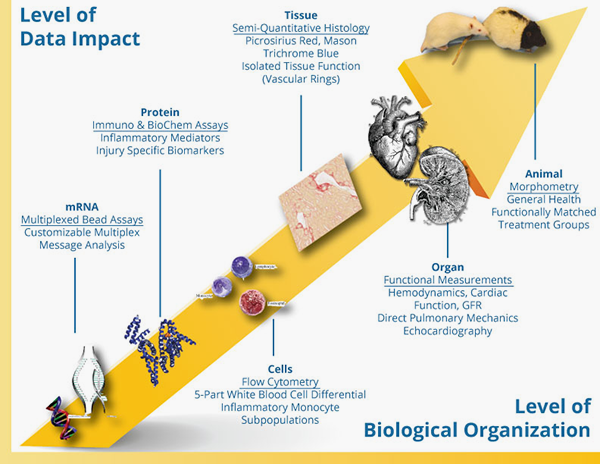
Biomarkers and In Vitro Biology


