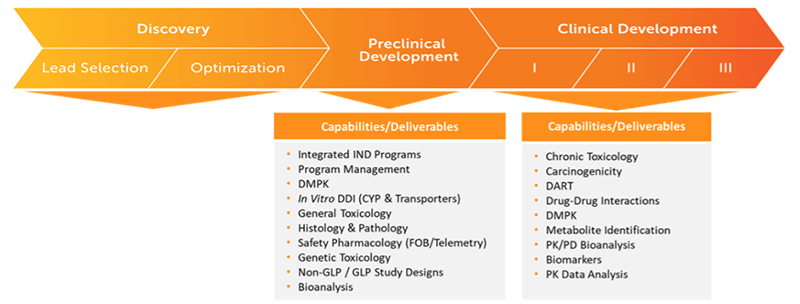
Seamlessly Moving Your Program From Discovery To Regulated Bioanalysis

Inotiv’s team of experts provide the resources, instrumentation, and technology needed to help navigate your molecule through the various stages of the drug development process. We offer a full array of services and capabilities to efficiently move you to first-in-human and beyond.
 As a proud winner of the Frost & Sullivan 2022 North American Bioanalytical Testing Services Customer Value Leadership Award, we are committed to delivering more attention, more insight, and a superlative client experience to help you advance to your next milestone.
As a proud winner of the Frost & Sullivan 2022 North American Bioanalytical Testing Services Customer Value Leadership Award, we are committed to delivering more attention, more insight, and a superlative client experience to help you advance to your next milestone.
To talk to an expert about our Bioanalytical Services, please click here.
Rapid Discovery LC-MS Bioanalysis
- Nonclinical and Early Phase Preclinical Programs and Studies
- Pharmacokinetic (PK) and Toxicokinetic (TK) Programs and Studies
- In Vitro and In Vivo Sample Analysis
- Stage-Appropriate Bioanalysis (Level 1 for screening; Level 2 for non-GLP IND-enabling)
- Tissue Bioanalysis
- Contemporary Bioanalytical Platforms and Technology
To learn more about our Nonregulated Discovery Bioanalytical services click here.
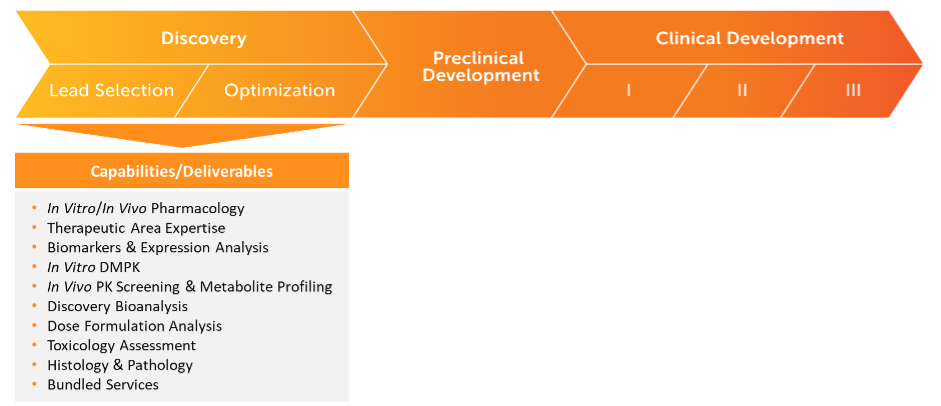
Preclinical Through Clinical LC-MS Bioanalysis
FDA, EMA, and OEDC compliancy
- Preclinical Programs and Studies
- Pharmacokinetic (PK) and Toxicokinetic (TK) Programs and Studies
- Clinical Study Support
- In Vitro Bioequivalence Study Support
- In Vitro and In Vivo Sample Analysis
- Tissue Bioanalysis
- Contemporary Bioanalytical Platforms and Technology
- Method Transfer(s)
- Method Development and Validation
To learn more about our Regulated Bioanalytical services click here.